How to design safe and sustainable chemicals
12 April 2022

In modern society, human-made chemicals are almost everywhere. You find them in food, clothes, toys, cosmetics, medicines and many more aspects of everyday life. Although developed for all kind of useful functions, these chemicals can at the same time possess hazardous properties that pose risks to public health and the environment. In many cases, these become only apparent a long time after their widespread use. The resulting environmental pollution is seen as a global threat and listed as one of the main drivers of biodiversity loss.
‘The problem with new chemicals is that their influx into the market far outpaces the speed with which hazard assessments can be performed’, says Joanke van Dijk, a PhD candidate at the Copernicus Institute for Sustainable Development at Utrecht University. In her research she aims to obtain insight into the future risks of chemicals, for which she cooperates with PhD candidates Hannah Flerlage and Steven Beijer and Dr Chris Slootweg at the Van 't Hoff Institute for Molecular Sciences at the University of Amsterdam (UvA). Van Dijk also investigates possible mitigation options in order to prevent chemical pollution of surface water, under the supervision of Prof. Annemarie van Wezel from the UvA Institute for Biodiversity and Ecosystem Dynamics.
‘Persistent chemicals can be an asset in a well-functioning circular economy, but chemicals should be designed for biodegradation when their release into the environment cannot be prevented’
Looking beyond a chemical’s function
According to Van Dijk, for many chemicals there is no adequate information on environmental hazards such as persistency and long-term effects. As a result, problems are often identified long after a chemical has been approved on the market. ‘To tackle this, the European Commission promotes the development of safe and sustainable chemicals as part of the European Green Deal’, Van Dijk says. ‘In our study, we have put these objectives into practice and developed a framework to design safe and sustainable chemicals. We assess whether a chemical can provide a certain function, but we look beyond that and provide an outlook on sustainability and hazards.’
In a case study, Van Dijk and co-workers focused on the organophosphate compound triisobutylphosphate (TiBP). As a flame-retardant this chemical contributes to protection against fire, but as a consequence of its widespread use it has been detected in many European waterbodies. ‘It leaches out of textiles during washing’, Flerlage explains, ‘so that it is released into the environment. As this release is inevitable, we chose to redesign TiBP in order to reduce its environmental persistence and improve its biodegradation.’
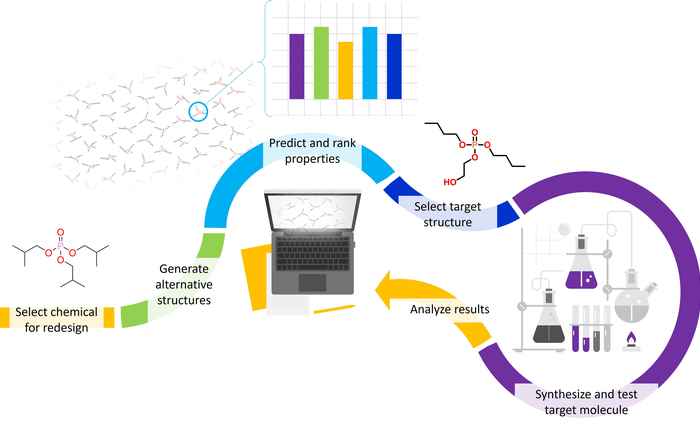
‘Persistent chemicals can be an asset in a well-functioning circular economy’, Flerlage adds. ‘But once released into the environment they are of major concern as they have the potential to affect organisms for a very long period of time. In order to prevent that, we have to redesign such essential chemicals to be biodegradable’.
Systematic redesign for safe chemicals
Van Dijk and Flerlage adapted a computer program to systematically generate over 6.3 million chemical structures similar to the original TiBP compound. Subsequently, they employed Quantitative Structure Activity Relationship (QSAR) modelling to predict the chemical properties relevant to the environmental fate and toxicity. All possible structures where then ranked, not only based on the environmental hazard properties but also on their ease of synthesis. This led to a 'top 500' of most benign structures that the researchers evaluated manually. They ultimately selected di-n-butyl (2-hydroxyethyl) phosphate as a target molecule, and synthesized this in the lab to confirm and complement the model predicted properties by experimental testing.
‘The first results indicate the flame-retardant function is preserved and possibly even enhanced’, Flerlage says. Although further testing is required to elucidate biodegradation mechanisms, the researchers are confident about their approach. ‘Experimental results such as this will help to expand and further verify our method, so that it can reach its full potential in the mitigation of chemical pollution and help enable a safe circular economy’, Van Dijk concludes.
Publication details
Joanke van Dijk, Hannah Flerlage, Steven Beijer, J. Chris Slootweg, Annemarie P. van Wezel: Safe and sustainable by design: A computer-based approach to redesign chemicals for reduced environmental hazards. Chemosphere 296: 134050 (June 2022). DOI: 10.1016/j.chemosphere.2022.134050