Chemical ecology laboratory

Wind tunnel
A large wind tunnel is available to measure insect flight behaviour and orientation responses to pheromones and plant odours. Orientation behaviour of smaller arthropods can be measured on an extra small "Kramer sphere", a locomotion compensator that allows the study of free walking insect down to the scale of mites.

Olfactometers
To quantify orientation responses of walking insects to odours, we use several types of olfactomters, such as Y-tube olfactometers and 4 choice olfactometers.

Headspace sampling
Sampling odours produced by insects and plants (headspace sampling) is a key step in chemo-ecological analysis. We use several multi-channel sampling systems to collect odours for behavioural and chemical analyses. In addition, we have a setup for quick and effective collection of pheromones from headspace with PDMS fibres (see Lievers & Groot, 2016).

Gas chromatography
We have gas chromatographs that are optimised for specific analyses: a fully automated system to analyse extremely small amounts of pheromone, and a system with a state of the art cold-on-column injector for high resolution and reduced discrimination of high boiling point compounds like cuticular hydrocarbons.
Electrophysiology lab
We have an electrophysiological setup with high quality optics to make single cells recordings (SSR), single unit recordings (tip recording), and electro-antennograms (EAG) from insect sensory organs.
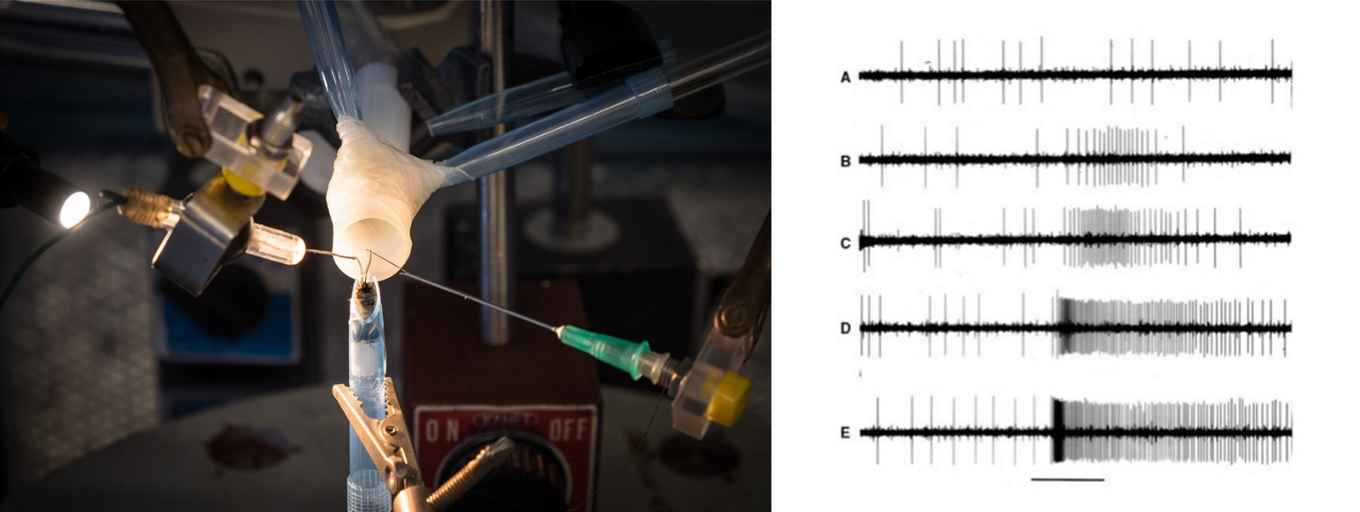

Electrophysiological measurements
Our lab is one of the few places in the Netherlands where gas chromatographic (GC) output is coupled with instruments for electrophysiological and behavioural research, particularly for the study of insect chemoreception and related fields. ElectroAntennography (EAG), Single Sensilum Recording (SSR) use the insect itself as a biological detector (GC-SSR and GC-EAD). In this setup we use a "Deans switch" that allows precise control of the split ratio between the flame ionisation detector in the GC and the insect’s antennal "detector".
Contact information
Please contact us for more information